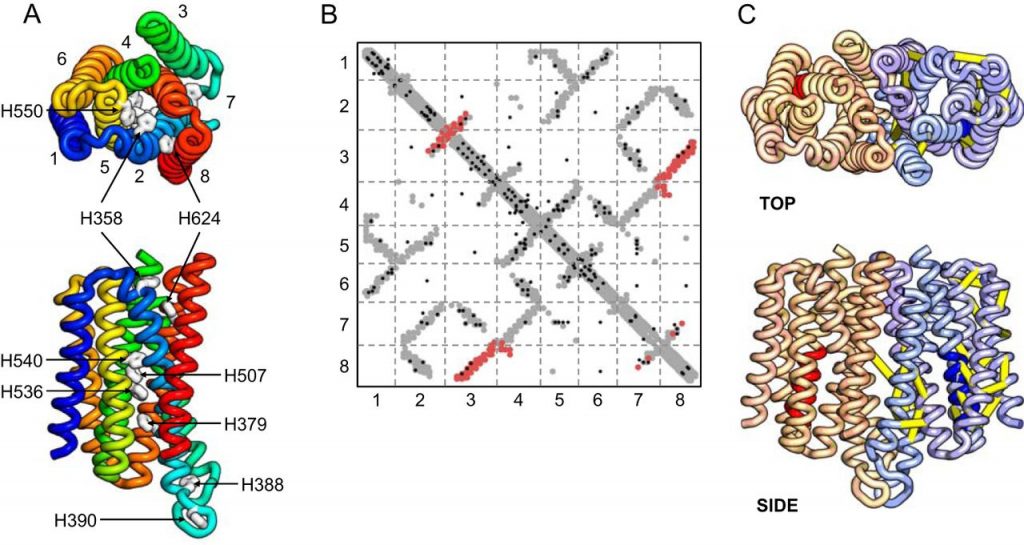
 First, we are interested in elucidating the molecular mechanism of the human (h) ZIP4 transporter (left), a protein which mediates zinc transport in vivo. hZIP4 was initially discovered as mutations in this protein result in acrodermatitis enteropathica, a childhood disease which is lethal when untreated. More recently hZIP4 has been implicated in solid tissue tumors such as pancreatic and breast cancer. Despite the central importance of this protein to cellular homeostasis, the mechanism of hZIP4 is not resolved.
First, we are interested in elucidating the molecular mechanism of the human (h) ZIP4 transporter (left), a protein which mediates zinc transport in vivo. hZIP4 was initially discovered as mutations in this protein result in acrodermatitis enteropathica, a childhood disease which is lethal when untreated. More recently hZIP4 has been implicated in solid tissue tumors such as pancreatic and breast cancer. Despite the central importance of this protein to cellular homeostasis, the mechanism of hZIP4 is not resolved.
Second, our laboratory is investigating the molecular components which define the unique functional properties of channelrhodopsin-2. While most opsin proteins are ion pumps, channelrhodopsin-2 is an ion channel, but can also pump protons. We use fluorescence spectroscopy in combination with two electrode voltage clamp and computational methods to examine the molecular details of the unique conformational dynamics of this protein which results in ion channel function. This enables our group to bypass purifying channelrhodopsin-2 while asking fundamental mechanistic questions.

